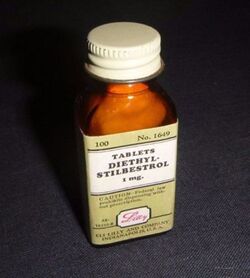
Diethylstilbestrol
(“Safe and Effective”, Drug) | |
|---|---|
 | |
| Start | 1938 |
| Given to pregnant women in 1938-1970s. Causes high risk of cancer and genital deformations for several generations. | |
A synthetic oestrogen known as Diethylstilbestrol (DES) was given to several million pregnant women to prevent miscarriage. The side effects of it have been passed on to daughters and granddaughters since the 1940s, in 'a scandal worse than Thalidomide'.[1]
It is thought maybe 8,000 British women were given DES during pregnancy, but because there are no records, the true scale is unknown. 300,000 received it the Netherlands, 200,000 in France. It was used widely in the US where five million are thought to have been exposed to the drug.
The deleterious effects of the drug on the offspring were irreversible for the generation of children exposed to DES in utero born between 1940 and 1980. The reproductive age for these children were mostly between 1975 and 2015.
Approval process
DES was created by British biochemist Sir Edward Charles Dodds in 1938 but he did not patent the product, which allowed more than 200 drug companies around the world to manufacture it. It was first prescribed by doctors for women who underwent repeated spontaneous abortions or premature deliveries. It also has some other uses (like prostate cancer). It was banned around the world in the 1970s.[1]
Dodds's experiments showed DES, which was known as Stilbestrol in the UK, could cause miscarriages in rabbits and rats. Despite this, in 1939 the UK Medical Research Council approved its use. [1]
Intergenerational cancer
Women who took the drug – 'DES mothers' – are thought to be 30 per cent more at risk of developing breast cancer.[1]
Their 'DES daughters' are 40 times more at risk of adenocarcinoma, a form of cervical cancer; eight times more likely to suffer neonatal death and almost five times more likely to have a premature birth.[1] Women exposed to the drug are also at risk of early menopause, infertility and ectopic pregnancies.
The sons of mothers who took the drug are at increased risk of infertility, micropenis and testicular cancer.[1]
In the years 2000-2010, data on the third human generation began to be sufficient to produce statistically significant study results. A Dutch study from 2007 found grandchildren at increased risk of some forms of severe esophageal malformation.[2]
Data published in 2010 also provide indications of an increased risk in girls and granddaughters of heart defects in granddaughters and boys. In November 2014, a study[3] concluded that the risk of inducing "genital anomalies" at birth were not increased in the 3rd generation of daughters, but in this 3rd generation, the risks of genital anomalies persist for boys.
References
- ↑ a b c d e f https://www.dailymail.co.uk/news/article-10363091/Shocking-health-timebomb-anti-miscarriage-drug-given-10-000-patients.html
- ↑ Felix JF, Steegers-Theunissen RP, De Walle HE, De Klein A, Torfs CP, Tibboel D, « Esophageal atresia and tracheoesophageal fistula in children of women exposed to diethylstilbestrol in utero » Am J Obstet Gynecol.
- ↑ Initiated by the D.E.S. France, funded by the National Medicines and Health Products Safety Agency (ANSM)